|
In a rush? A quick summary on Binding energy, useful for review Otherwise scroll down |
|
Imagine a lego kit. You weigh the parts and get a certain mass.
You complete the project and when you weigh the final project, its less than what you started with.
But you haven't lost a piece!
In fact, when you pull the whole project apart , all the parts have the same mass you initially measured.
This analogy describe the nucleus and the idea of mass defect and binding energy.
Before we explain binding energy lets first look at mass defect.
You complete the project and when you weigh the final project, its less than what you started with.
But you haven't lost a piece!
In fact, when you pull the whole project apart , all the parts have the same mass you initially measured.
This analogy describe the nucleus and the idea of mass defect and binding energy.
Before we explain binding energy lets first look at mass defect.
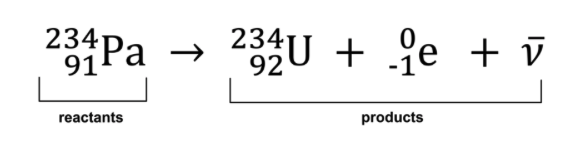
Mass Defect
When nucleus transmutes, such as in the case of alpha or beta decay, energy is released. But where does that energy come from? (It also occurs in nuclear fission and fusion, which we will discuss shortly)
The answer simply is from the matter itself.
Like a chemical reaction where you have reactants and products, so too, in nuclear reactions you have a reactant or reactants which results in the production of the products.
During the process of the nuclear reaction there is less mass in the products than the reactants.
This seems to violate one of the conservation laws: one of the conservation of matter. But the fact is the mass lost is converted into energy - the mass defect.
Some more correctly the conservation laws is about the conservation of mass-energy, mass is just the concentration of energy by way of E=mc^2
This is referred to as the mass defect. And it is that mass difference that converts to energy by way of Einstein's famous equation.
When nucleus transmutes, such as in the case of alpha or beta decay, energy is released. But where does that energy come from? (It also occurs in nuclear fission and fusion, which we will discuss shortly)
The answer simply is from the matter itself.
Like a chemical reaction where you have reactants and products, so too, in nuclear reactions you have a reactant or reactants which results in the production of the products.
During the process of the nuclear reaction there is less mass in the products than the reactants.
This seems to violate one of the conservation laws: one of the conservation of matter. But the fact is the mass lost is converted into energy - the mass defect.
Some more correctly the conservation laws is about the conservation of mass-energy, mass is just the concentration of energy by way of E=mc^2
This is referred to as the mass defect. And it is that mass difference that converts to energy by way of Einstein's famous equation.
|
But in nuclear physics, is is more helpful to to use a non SI unit for mass, as well as for energy
So instead of using the joule (J) for energy we can use the electron volt (eV) And for mass , instead if using the kilogram, we use the atomic mass unit (u). The video covers this Before you continue however, make sure you are familiar with the electron volt (eV) as a unit of energy - If not, please review here. |
|
Binding Energy
Now that we understand the mass defect, discuss move on to the concept of Binding energy.
What if we wanted to MAKE a chlorine atom?
We would have 17 protons, 17 electrons and 18 neutrons. But if we added their masses we would get a total mass that is greater than the mass of a chlorine atom. If still have a mass defect!
Watch this video as we tie the mass defect to Binding energy.
(this video does covers the atomic mass unit which you can watch for review (and helpful if you are still a little unsure) , but if you wish to go straight to Binding Energy , scroll to 3:25)
Now that we understand the mass defect, discuss move on to the concept of Binding energy.
What if we wanted to MAKE a chlorine atom?
We would have 17 protons, 17 electrons and 18 neutrons. But if we added their masses we would get a total mass that is greater than the mass of a chlorine atom. If still have a mass defect!
Watch this video as we tie the mass defect to Binding energy.
(this video does covers the atomic mass unit which you can watch for review (and helpful if you are still a little unsure) , but if you wish to go straight to Binding Energy , scroll to 3:25)
Sample Problem
We are now ready to try a sample problem
Below is a sample problem with a video that explain how to solve it. It is suggested you try the problem beforehand, as this actually aids understanding, even if you are unsure if you are correct.
We are now ready to try a sample problem
Below is a sample problem with a video that explain how to solve it. It is suggested you try the problem beforehand, as this actually aids understanding, even if you are unsure if you are correct.
Some more problems
Tritium is an isotope of hydrogen. The mass of the tritium isotope, H-3, is 3.0160490 u.
Tritium is an isotope of hydrogen. The mass of the tritium isotope, H-3, is 3.0160490 u.
- What is the mass defect of this isotope? (0.009106 u)
- What is the binding energy of this isotope? (8.48 MeV)
- Find the binding energy per nucleon. (2.83 MeV)
- What is the mass defect of this nucleus? (1. 0.98940 u)
- What is the binding energy of this nucleus? (92.1 MeV)
- Find the binding energy per nucleon. (7.68 MeV)

